veriseq nipt v2
VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. Product includes components of library preparation sequencing and analysis.

The Veriseq Nipt Solution Youtube
Download 1 MB Dec 8 2017 IVD Symbol Key.

. The BaseSpace RNA-Seq Alignment App analyzes data from the TruSight RNA Pan-Cancer Panel providing a simple results summary that includes a fusion table variant table and gene expression table. VeriSeq NIPT v2 offers superior performance to any IVD noninvasive prenatal testing solution available and genome-wide coverage for in-lab offerings. The test offers an option to request the reporting of sex chromosome aneuploidy SCA.
You can also use your own pipeline for analysis. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and deletions for all autosomes and aneuploidy status for all chromosomes. Elevating the standard for prenatal screening Noninvasive NIPT is a simple blood draw with little risk to the fetus or the patient Accurate Illumina NIPT shows 99 sensitivity and specificity for trisomies 13 18 21 1 Early.
Here archived plasma samples were used. The laboratory can choose to run basic or ge- nome-wide screening by sample. Đổi tên đợt thành tên không chứa ký tự văn bản đặc biệt nào.
Hands-free throughput and sample integrity are enabled through powerful features such as eight. Skip to content ProductsLearnCompanySupportRecommended Links Products Instruments Kits Reagents Selection Tools Software Analysis Services Popular Products Explore All Products Instruments. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
Health Care Served as technical writing project lead for the release and launch of VeriSeq NIPT Solution v2 an end-to-end solution for non-invasive prenatal testing. Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. CfDNA was extracted from 1 mL of plasma by adsorption onto a binding plate to remove.
VeriSeq NIPT Solution v2 chỉ chấp nhận số chữ cái dấu gạch dưới và dấu gạch ngang cho tất cả các trường dữ liệu. View Options IVD Symbol Key Symbol key and translations for Illumina IVD products. The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for.
View Options VeriSeq NIPT Solution v2 Software Guide Instructions for use of the software involved with the VeriSeq NIPT Solution v2. The automated workflow easily scales to analyze 24 48 or 96 samples per run to allow for efficiency and flexibility in managing sample volumes. In a clinical setting plasma would be isolated from 710 mL of whole blood from pregnant women 10 weeks gestation.
This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. Using Illuminas VeriSeq NIPT Solution v2 the test delivers a comprehensive view of the fetal genome compared to other CE-IVD NIPT products enabling healthcare providers to support expectant.
VeriSeq NIPT Solution v2 incorporates automated sample preparation and sequencing data analysis. PDF 1 MB Aug 13 2021. EM0051 The Batch ID is greater than 26 characters in length.
VeriSeq NIPT Solution v2 is a next-generation sequencing based method to noninvasive prenatal testing Illuminas VeriSeq NIPT Solution v2. Download 1 MB Apr 19 2017 VeriSeq NIPT Solution Consumables Equipment List Interactive list of consumables and equipment used with the VeriSeq NIPT Solution kit. RevisionHistory Document Date DescriptionofChange Document 1000000067940v06 August 2021 UpdatedEUAuthorizedRepresentativeaddress.
All Reproductive Health Products. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. VeriSeq NIPT Solution Sample Prep Checklist A condensed version of the VeriSeq NIPT Sample Preparation kit protocol for experienced users.
The assay provides information about fetal chromosomal status as early as 10. ID đợt dài hơn 26 ký tự. Das von uns verwendete CE-zertifizierte Testverfahren VeriSeq NIPT Solution v2 von Illumina ergab in einer klinischen Studie mit über 2200 Proben für die autosomalen Trisomien 13 18 und 21 eine Entdeckungsrate von jeweils über 99.
VeriSeq NIPT Solution Comprehensive and reliable NIPT solution Reagents instruments and CE-IVD marked library prep and analysisreporting software in an automated workflow for in-lab prenatal aneuploidy screening. The integrated VeriSeq NIPT Solution v2 provides every - thing needed to run the assay. Enhanced Time Savings and Sample Integrity.
Sequencing with the VeriSeq NIPT Solution v2 enables comprehensive insights reducing the need for invasive tests. Noninvasive Prenatal Testing NIPT NGS-based technology Expect the extraordinary NIPT. This noninvasive test provides an option.
Created edited and updated the Package Insert and Software Guide for the product. The VeriSeq NIPT Microlab STAR allows a single user to prepare and analyze 48 or 96 samples simultaneously with results in approximately one day compared to two days or longer using other methods. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw.
Run the RNA-Seq workflow FASTQ only on the MiSeq and stream the data to BaseSpace. This product must not be used as the sole basis for diagnosis or other pregnancy management decisions. ProductsLearnCompanySupportRecommended Links Products Software Analysis Services Popular Products Instruments Selection Tools.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. VeriSeq NIPT Solution v2.
Der NIPT erkennt die Trisomien 13 18 und 21 mit einer sehr hohen Wahrscheinlichkeit. Business Wire Illumina has collaborated with Next Generation Genomic NGG Thailand to introduce an automated in-lab IVD solution called VeriSeq NIPT Solution v2 in Thailand. Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor.

Veriseq Nipt Solution V2 Support

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
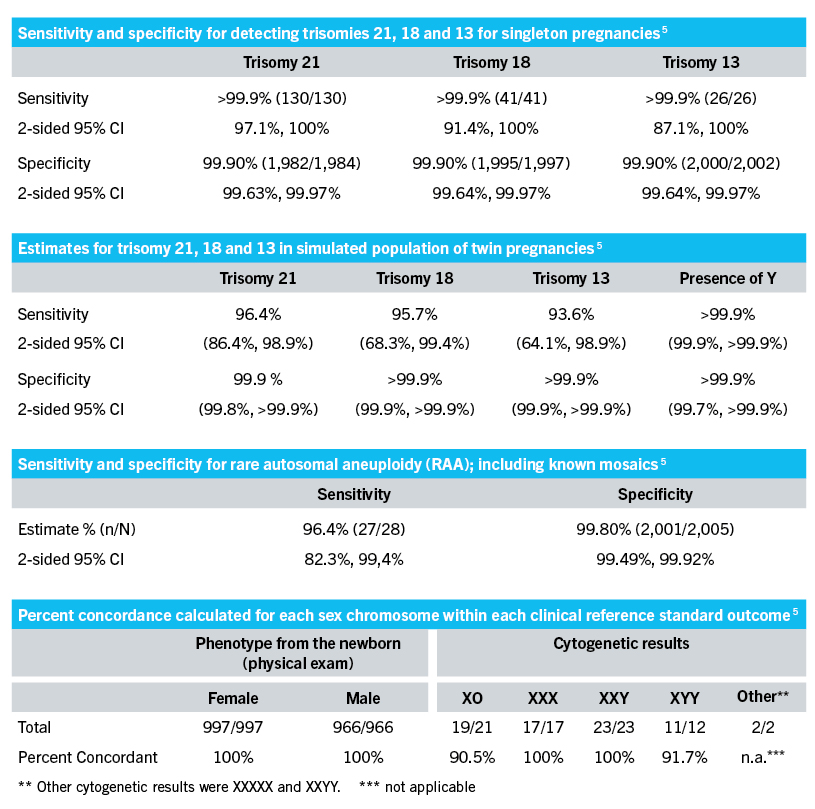
Performance Qualification Praenatest
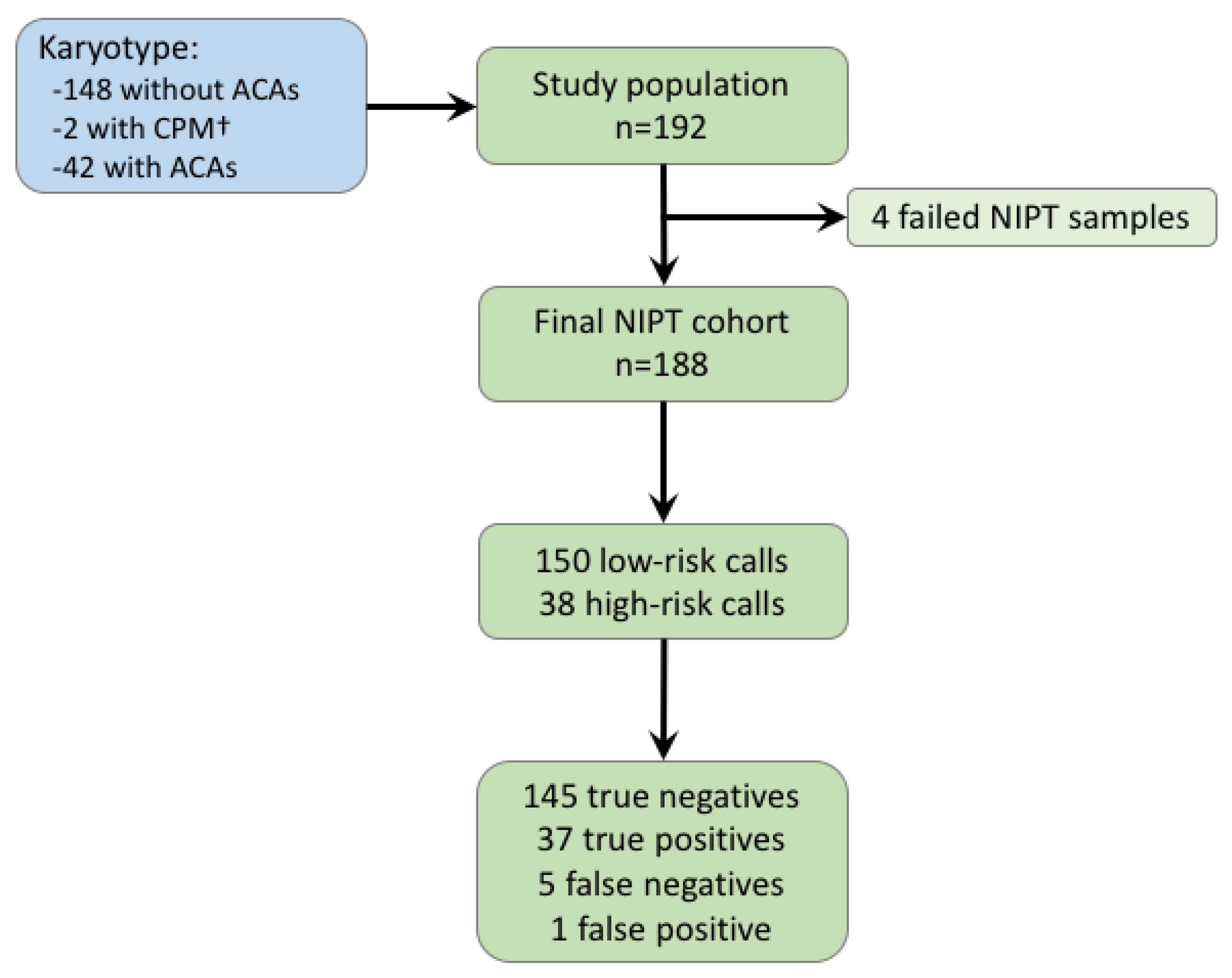
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire
Illumina 簇生成和测序试剂 Fc 126 1003illumina Fc 126 1003 上海易汇生物科技有限公司

Illumina On Twitter Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
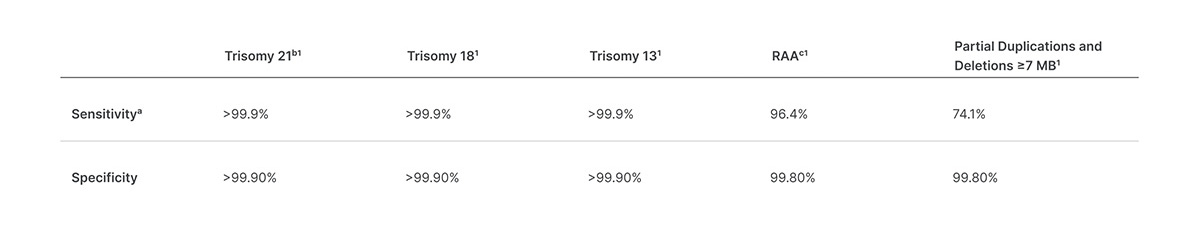
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Comparison Of Aneuploidy Incidence Between Study Cohorts Download Table
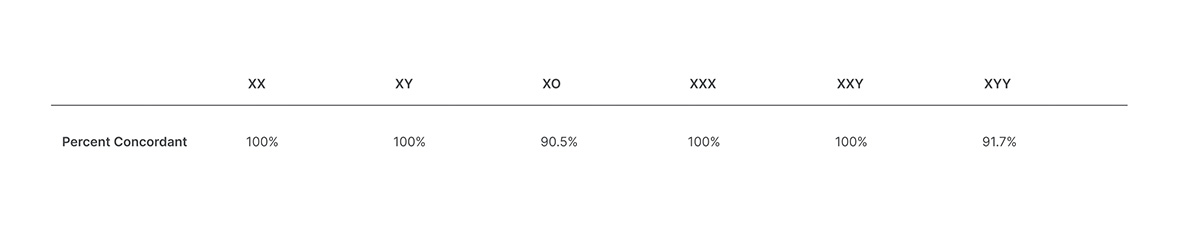
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Analysis Of Cfdna Using A Modified Illumina Veriseq Non Invasive Download Scientific Diagram

Veriseq Nipt Solution V2 Genetica
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution


Comments
Post a Comment